Article

Despite careful preparation, unforeseen issues can arise in the course of a clinical trial. When a trial finds itself in need of rescuing, it is critically important that the chosen intervention swiftly and adeptly gets the trial back on its feet.
So, why do some clinical trials find themselves in need of rescuing? Sometimes it’s a matter of resource shortages, either internal or external. Other times it’s the derailment of a study timeline due to the need for additional clinical services that are necessary but unaccounted for at the start of a project. It can even be as simple as another contracted party becoming unable to fulfill expectations.
Whatever the case, it’s nearly impossible to plan for every possible factor that could disrupt the course of a clinical trial – and sometimes despite the best-laid plans, a trial finds itself in need of a rescue party.
When it comes to planning a clinical trial rescue study, early intervention is key. It is likely that significant delays in the clinical trial process have already taken place, and you want to ensure you regain as much momentum as you can as quickly and efficiently as possible. However, it is paramount to have a clear understanding of the trial process as a whole, and exactly what aspects of the clinical trial are in need of rescuing. As you begin to plan your rescue study transition, keep these steps in mind.
When researching rescue CROs, look for a central lab that will be able to meet the unique needs of your rescue study. No two interventions are the same, as every clinical trial rescue study is unique to the needs of the client, and the intervention utilized should be able to meet the unique needs of the trial in question.
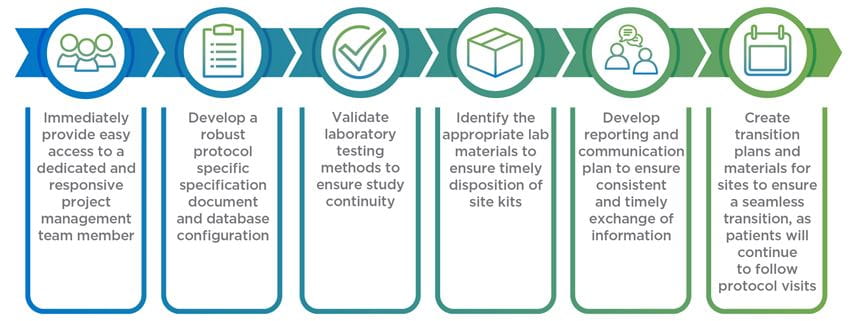
Once you have a plan designed for how your clinical trial needs to be rescued, the next step is determining what rescue CRO you are going to utilize to get your trial back where it needs to be. To help ensure a seamless transition, you should provide any potential rescue CROs with this information:
We partner with you to make sure we’re the right fit as we work to align the right resources to get your clinical study back on track and deliver according to submission timelines.
This includes:
At ACM, we understand that each study is unique and therefore every rescue study must be carefully crafted to fill distinctive needs.
As such, the Rescue Team at ACM will conduct a thorough review of your current study to develop a customized plan to course correct, with a careful focus on the quality and expertise your study demands.
The Rescue Team will review the core issue(s), conduct a root cause analysis, assess the management of vendors and review all necessary data in assembling a corrective and preventative plan to ensure your goals are met.
No one wants to find themselves in need of a rescue study, but nevertheless, these things can and do happen. When they do, ACM is ready and able to provide the highest level of guidance with a personal approach, to ensure that your study gets back on track quickly and efficiently.
With our global footprint and decades of successful clinical trial and rescue study experience, we are an honest and accountable lab that values flexibility, communication, and transparency. No matter where you are in the lifecycle of your trial, ACM has the expertise and ability to step in at any point to get your study back on track.
Whether you are experiencing issues in your current study with regard to compliance, quality, resources, and/or timelines, ACM Global Laboratories offers a comprehensive rescue service for any aspect of your clinical trial - no matter the stage.
To discover strategies for how to rescue a clinical study or delve deeper into our Rescue Study services, get in touch with ACM today.