Article

The pharmaceutical industry is undoubtedly one of the most dynamic from an R&D perspective, with hundreds of new therapeutics entering the market every year, each undergoing a rigorous process and path to approval.
Advancements in technology, expertise and dedication from countless organizations across the globe have changed the financial forecast of clinical trials over the course of a decade. The true cost of clinical trials will continue to be a contentious topic, so we dove into a few contrasting findings to get a better look at what CROs and sponsors might expect to invest.
Total life cycle costs of developing and gaining marketing approval for a new prescription drug once hovered around $3 billion, according to a 2014 study by the Tufts Center for the Study of Drug Development (CSDD). In that same Tufts study, which was based on information provided by 10 pharmaceutical companies on 106 randomly selected drugs with FIH subjects between 1995 and 2007, researchers then estimated the average cost of a Phase III trial to be in the ballpark of $255 million.
More recently, however, a June 2021 British Medical Journal article featured a new study conducted by the Institute for Safe Medication Practices (ISMP), which pulled data from 101 new drugs approved from 2015 to 2017 and concluded the median cost for a clinical trial averaged $48 million, with an interquartile range (IQR) of $20 million to $102 million. In the ISMP study, it was found that the cost per drug varied, with 45 of the 101 drugs analyzed approved with a single trial (median cost $28 million), whereas 29 of the reviewed treatments had double the cost when two trials were required (median cost $45 million). Those drugs requiring three to eleven trials had an estimated median cost of $91 million.
Of course, there is no one-size-fits-all equation when it comes to clinical trials, and there are many factors that influence the cost of a trial. For example, in the ISMP study, the number of patients required to establish the treatment effects varied from four to 8,442, and the number of clinic visits patients had to make ranged from two to 166. Of the 62 trials in which there was an existing active drug being used as a control group, there was a median of 653 enrolled patients, compared to other studies which utilized a placebo (547) and those which were controlled (145).
Another element impacting overall estimated trial costs include the number of necessary clinic visits, which, according to the ISMP study, average a median of 11, with each additional visit costing in the neighborhood of $2 million. Essentially, the ISMP study found that the estimated trial cost per patient average came to about $41 thousand (IQR of $29,894 to $75,047) and each patient visit to a clinic cost an average of $3,685 (IQR of $2,640 to $5,498).
Trial costs will also vary depending on the therapeutic area, as treatments vary from topicals to infusions and other intensive treatments. The increased cost of goods and services will also undoubtedly continue to tax the industry, as well as ever-changing regulations. A study published by JAMA International Medicine in 2018 found that out of 138 pivotal trials assessing 59 new therapies that received FDA approval (between 2015 and 2016), there was a more than 100-fold difference in the cost of clinical trials.
The rise of consolidated CRO-giants has changed the market landscape, and because of this, sponsor companies need to be even more vigilant in their planning. It’s difficult to put a price tag on developing life-saving therapeutics to treat patients around the world, however, there are ways to mitigate surplus expenditures.
ACM Global Laboratories’ expansive and inclusive executive and project management teams are built with CRO and sponsor fiscal goals in mind. We make it our business to understand the various cost drivers and parameters of clinical trials – no matter the phase or complexity.
There are many, but here are the most common our experts see at ACM:
Knowing some of the key drivers of cost with regard to clinical trials, the experienced team at ACM Global Laboratories can help build the most effective and efficient central lab budget needed to support the trial in question. Whether the study requires a mix of routine safety, flow cytometry, microbiology, pathology or bioanalysis, ACM is uniquely, and globally positioned to maximize investments.
When it comes to building the central lab budget, our team will consider a host of elements to maximize the dollar, which starts with the utilization of our global network of partner labs. From there, we look at a few integral elements including lab specialties, kitting, sample logistics, storage and data management.
As an industry-leading global central lab, our project management teams cover budgeting estimations on a granular level, with a hyper-focus on building out specimen management and Pharmacokinetic (PK) needs. When it comes to developing budgetary line items around PK requirements, we’ll dig into the following:
Our reputation for building long-standing client and partner relationships comes from the unsurpassed service we provide. Our consultative approach gives our customers the confidence to make informed decisions and meet every study’s objectives. We can help navigate the complexities of transporting samples globally by validating compliance with local regulations, managing couriers and shipments and providing shipping options that optimize cost and efficiency. Having a dedicated project manager from project inception to completion isn’t just a nice to have, it’s a necessity, and helps to ensure a properly executed protocol.
Maintaining a global footprint with labs in the US, UK, and APAC isn’t a task we take lightly. Each headquartering lab has its own delegation and responsibilities, and when it comes to specimen management, ACM has it down to a science, and utilizes a number of harmonization tools including our newly-refreshed global Client Portal, which manages sample data and kitting supplies.
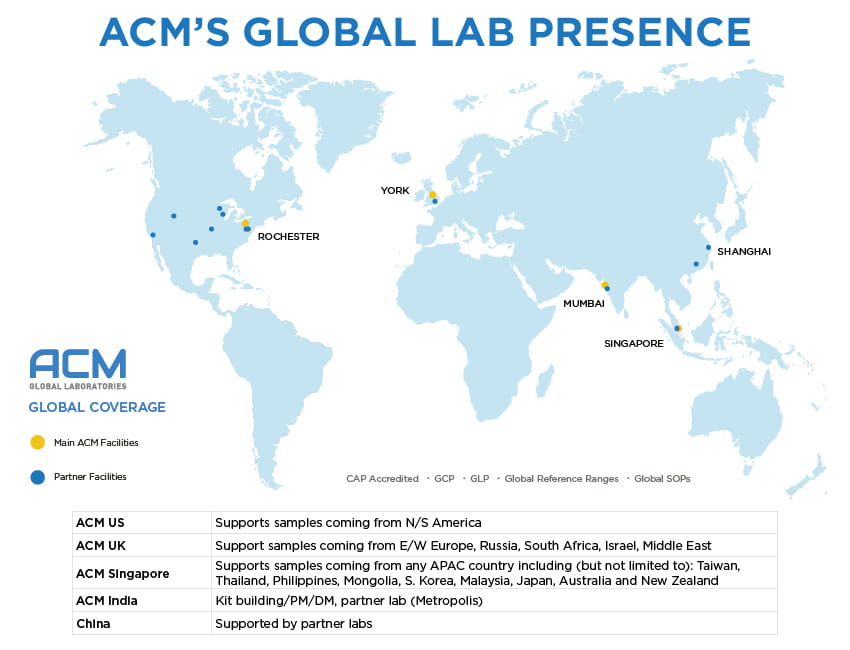
Our dedicated logistics team communicates with clients and couriers on a multiple-times-daily basis, ensuring our clinical specimen collection kits are on location, on time, and specimens are routed appropriately. Executing the leg work prior to study kickoff is key, and our teams will leave no stone unturned, minimizing surprise costs down the road. Each clinical trial program must be customized to address unique specifications and challenges. By partnering with ACM Global Laboratories, our scientific experts, project managers, and other professionals are available to help you obtain the information needed to make data-driven decisions about your clinical trials program from inception to completion – all within budget.
To learn more about how ACM can support your next study, contact us today.