Article
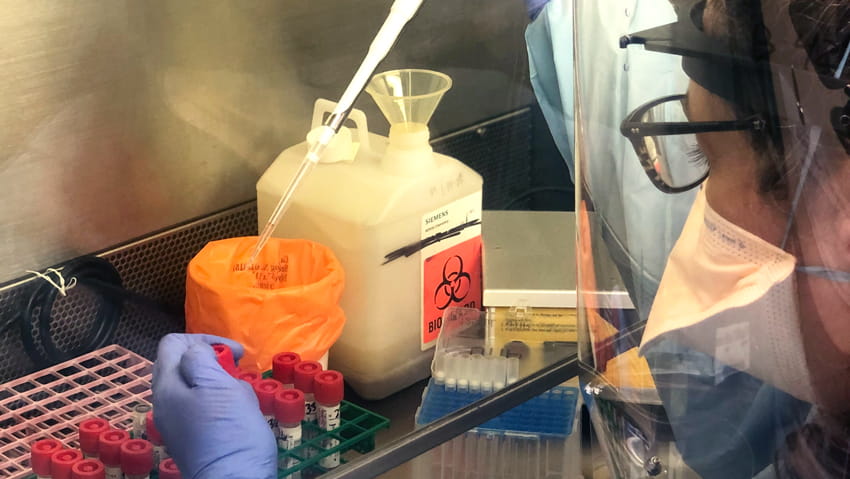
As a provider of central laboratory testing for clinical trials, a focus on stringent laboratory safety standards is a commitment that ACM Global Laboratories shares with the entire healthcare industry. Following biosafety standards mandated by the Occupational Safety and Health Administration (OSHA), US Centers for Diseases Control and Prevention (CDC), and the World Health Organization is vital for limiting exposure to infectious diseases.
Every day, our dedicated laboratories adhere to best practices that minimize their risk of coming into contact with infectious microbes that may be present in blood, plasma, urine, feces, and other specimen types. It must be assumed that all biological samples are potentially hazardous.
As concerns about environmental health and safety measures became heightened as a result of the novel coronavirus (COVID-19) outbreak, questions arose about the best practices and PPE needed to maintain productivity in the central lab while simultaneously keeping employees safe from a new pathogen that the medical and scientific communities were just beginning to understand. Do some specimens pose more of an exposure risk to SARS-CoV-2, the virus that causes COVID-19, than others? What biosafety level was needed to protect against exposure to the novel virus?
Questions around effective social distancing measures were also raised. To address these concerns, The ACM COVID-19 Task Force worked closely with the Systems Incident Command Center from Rochester Regional Health in Rochester, New York to perform thorough risk assessments and gather occupational safety and health information from the CDC and New York State Health officials.
ACM Global Lab’s environmental health & safety response to the novel coronavirus
Since the virus can cause a respiratory tract infection, ACM implemented enhanced universal laboratory safety procedures that— in addition to biosafety level 2 requirements already established at our lab facilities— include extra precautions for handling upper and lower respiratory tract specimens. The following information outlines standard universal lab safety procedures and enhanced precautions for clinical laboratory employees at ACM Global and Rochester Regional Health Laboratories.  Physical distancing measures for increasing workplace safety during the pandemic
Physical distancing measures for increasing workplace safety during the pandemic
While simultaneously implementing enhanced biosafety procedures, ACM ascertained the appropriate approach to maximize best practices for physical distancing and increasing health and hygiene practices in a laboratory setting.
It was necessary to perform individual risk assessments for each of our global facilities and adopt policies that accounted for the number of employees at each site and building size and configuration. In some cases, for example, shift work was implemented to limit the density of people in laboratory or processing spaces at any one time. At larger facilities, shift work was not required, as it was possible to space work stations to adequately ensure physical distancing. Each of the following measures went into effect to protect employees, and these practices are continually monitored and updated as health officials refine guidelines and regulations.
Current Physical Distancing, Health, and Hygiene Measures at ACM Facilities
By proactively taking these steps in addition to stringently observing enhanced biosafety precautions, our company has maximized the level of precautions at all ACM facilities.

Safeguarding our employees and the communities where they work has always been of paramount importance at ACM Global. With the aid of seasoned professionals, we are remaining well-informed and ready to adopt public health measures in an ever-changing landscape. As new guidance related to COVID-19 or other potential health threats emerge, our company will continue to onboard best practices in the interest of supporting employees and our valued clients.
The global response to COVID-19 has further raised public health awareness and has increased the resolve of clinical laboratories to build upon mandatory lab safety monitoring practices. Ultimately, these changes benefit the healthcare industry as a whole.